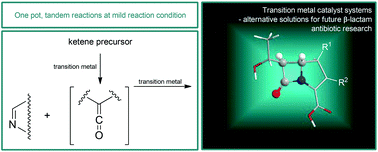
Synthesis of β-lactams by transition metal promoted Staudinger reactions: alternative synthetic approaches from transition metal enhanced organocatalysis to in situ, highly reactive intermediate synthesis and catalytic tandem reactions†
Abstract
The development of new types of β-lactam

 Please wait while we load your content...
Please wait while we load your content...