Orthogonally protected d-galactosamine thioglycoside building blocks via highly regioselective, double serial and double parallel inversions of β-d-thiomannoside†
Abstract
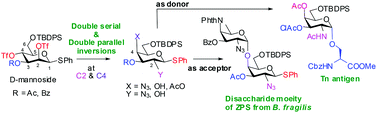
An efficient route for the synthesis of orthogonally protected D-galactosamine thioglycosides via one-pot double serial and double parallel displacements of the D-mannosyl-2,4-bis-trifluoromethanesulfonates by azide and

 Please wait while we load your content...
Please wait while we load your content...