Straightforward synthesis of non-natural l-chalcogen and l-diselenide N-Boc-protected-γ-amino acid derivatives†
Abstract
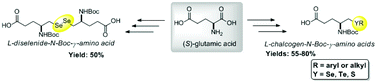
The synthesis of new chiral seleno-, telluro-, and thio-N-Boc-γ-amino acids is described herein. These new compounds were prepared through a simple and short synthetic route, from the inexpensive and commercially-available amino acid L-glutamic acid. The products, with a highly modular character, were obtained in good to excellent yields, via

 Please wait while we load your content...
Please wait while we load your content...