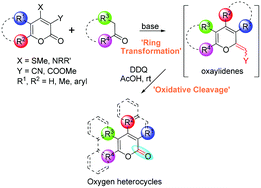
Diversity-oriented general protocol for the synthesis of privileged oxygen scaffolds: pyrones, coumarins, benzocoumarins and naphthocoumarins†
Abstract
A new general methodology for the synthesis of various functionalized privileged oxygen heterocyclic scaffolds, viz. pyrones,

 Please wait while we load your content...
Please wait while we load your content...