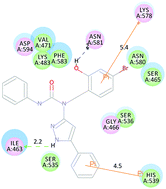
The RAF–MEK–ERK cascade appears to be intimately involved in the regulation of cell cycle progression and apoptosis. The BRAFV600E mutant results in constitutive activation of the ERK pathway, which can lead to cellular growth dysregulation. A series of 5-phenyl-1H-pyrazol derivatives (3a–5e) have been designed and synthesized, and their biological activities were evaluated as potential BRAFV600E inhibitors. All the compounds were reported for the first time except 3e, and compound 1-(4-bromo-2-hydroxybenzyl)-3-phenyl-1-(5-phenyl-1H-pyrazol-3-yl)urea (5c) displayed the most potent inhibitory activity (BRAFV600E IC50 = 0.19 μM). Antiproliferative assay results indicated that compound 5c possessed high antiproliferative activity against cell lines WM266.4 and A375 in vitro, with IC50 values of 1.50 and 1.32 μM, respectively, which were comparable with the positive control vemurafenib. Docking simulations showed that compound 5c binds tightly to the BRAFV600E active site and acts as BRAFV600E inhibitor. A 3D-QSAR model was also built to provide more pharmacophore understanding towards designing new agents with more potent BRAFV600E inhibitory activity.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...