One-pot synthesis of S-alkyl dithiocarbamates via the reaction of N-tosylhydrazones, carbon disulfide and amines†
Abstract
A new, convenient and efficient transition metal-free synthesis of S-alkyl dithiocarbamates through one-pot reaction of N-tosylhydrazones, carbon disulfide and
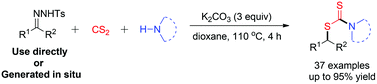

 Please wait while we load your content...
Please wait while we load your content...