Synthesis of functionalised 4H-quinolizin-4-ones via tandem Horner–Wadsworth–Emmons olefination/cyclisation†
Abstract
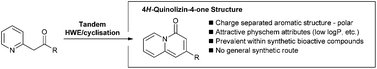
4H-Quinolizin-4-ones are a unique class of heterocycle with valuable physicochemical properties and which are emerging as key pharmacophores for a range of biological targets. A tandem Horner–Wadsworth–Emmons olefination/cyclisation method has been developed to allow facile access to substituted 4H-quinolizin-4-ones encoded with a range of functional groups.

 Please wait while we load your content...
Please wait while we load your content...