Symmetric and unsymmetric 3,3′-linked bispyrroles via ring-enlargement reactions of furan-derived donor–acceptor cyclopropanes†
Abstract
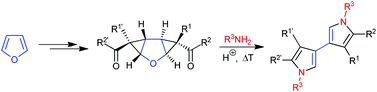
A short and concise sequence for the synthesis of symmetrically and unsymmetrically substituted 3,3′-linked bispyrroles is described. Furan as a starting material is subjected to a twofold cyclopropanation by a diazo ester. Conversion of the ester functionalities to the respective ketones is achieved via Weinreb amide formation and the attack of a Grignard reagent. In the presence of amines the ketone moieties form imines that rearrange by ring-enlargement of the three-membered to the five-membered rings. In situ generated dihydropyrrole moieties eliminate water and afford aromatic pyrrole units. Unsymmetric bispyrroles are obtained by using different cyclopropanating agents or varying Grignard reagents for ketone formation before the addition of amines initiates the cascade to the respective bispyrroles.

 Please wait while we load your content...
Please wait while we load your content...