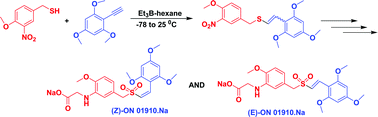
Hydrothiolation of benzyl mercaptan to arylacetylene: application to the synthesis of (E) and (Z)-isomers of ON 01910·Na (Rigosertib®), a phase III clinical stage anti-cancer agent†
Abstract
A stereoselective and efficient method for free radical addition of

 Please wait while we load your content...
Please wait while we load your content...