Synthesis of novel heterocyclic oleanolic acid derivatives with improved antiproliferative activity in solid tumor cells†
Abstract
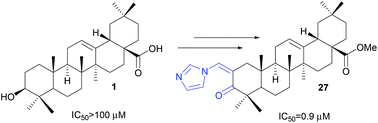
A series of new oleanane imidazole carbamates,
* Corresponding authors
a
Laboratório de Química Farmacêutica, Faculdade de Farmácia da Universidade de Coimbra, Poló das Ciências da Saúde, Azinhaga de Santa Comba, 3000-548 Coimbra, Portugal
E-mail:
salvador@ci.uc.pt
Fax: +351-239-488-503
Tel: +351-239-488-400
b CNC-Centro de Neurociências e Biologia Celular, Universidade de Coimbra, 3000-517 Coimbra, Portugal
c
Division of Hematology/Oncology, The Tisch Cancer Institute, Mount Sinai School of Medicine, New York, 10029 NY, USA
E-mail:
yongkui.jing@mssm.edu
Fax: +1-212-996-5787
Tel: +1-212-241-6775
A series of new oleanane imidazole carbamates,

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
A. S. Leal, R. Wang, J. A. R. Salvador and Y. Jing, Org. Biomol. Chem., 2013, 11, 1726 DOI: 10.1039/C3OB00011G
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
