A concise one-pot synthesis of trifluoromethyl-containing 2,6-disubstituted 5,6,7,8-tetrahydroquinolines and 5,6,7,8-tetrahydronaphthyridines†
Abstract
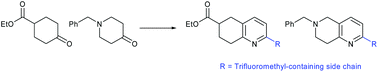
5,6,7,8-Tetrahydroquinolines and 5,6,7,8-tetrahydronaphthyridines with appended trifluoromethyl groups are valuable chemotypes in medicinal chemistry due to the presence of a partially-saturated bicyclic ring and metabolically-stable CF3 group. 1H NMR studies were used to optimize the preparation of such compounds, using a three-step/one-pot procedure, to provide novel 2,6-disubstitued derivatives with a tertiary-substituent. Racemic 2,6-disubstituted tetrahydroquinolines were separated by chiral HPLC to provide single enantiomers.

 Please wait while we load your content...
Please wait while we load your content...