Synthesis and stereochemical analysis of β-nitromethane substituted γ-amino acids and peptides†
Abstract
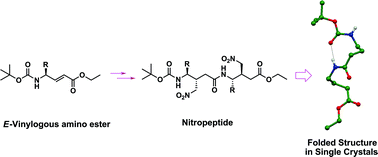
The high diastereoselectivity in the Michael addition of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Cα eclipsed conformer of the unsaturated amino ester leads to the major (anti) product compared to that of an N–Cγ–Cβ
Cα eclipsed conformer of the unsaturated amino ester leads to the major (anti) product compared to that of an N–Cγ–Cβ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Cα eclipsed conformer. The major diastereomers were separated and subjected to the
Cα eclipsed conformer. The major diastereomers were separated and subjected to the

 Please wait while we load your content...
Please wait while we load your content...