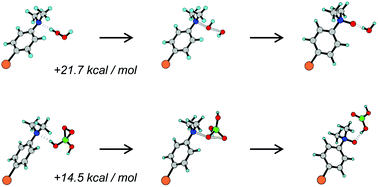
Our recent work has provided new insights into the equilibria and species that exist in aqueous solution at different pHs for the boric acid – hydrogen peroxide system, and the role of these species in oxidation reactions. Most recently, (M. C. Durrant, D. M. Davies and M. E. Deary, Org. Biomol. Chem., 2011, 9, 7249–7254), we have produced strong theoretical and experimental evidence for the existence of a previously unreported monocyclic three membered peroxide species, dioxaborirane, that is the likely catalytic species in borate mediated electrophilic reactions of hydrogen peroxide in alkaline solution. In the present paper, we extend our study of the borate–peroxide system to look at a wide range of substrates that include substituted dimethyl anilines, methyl-p-tolyl sulfoxide, halides, hydrogen sulfide anion, thiosulfate, thiocyanate, and hydrazine. The unusual selectivity–reactivity pattern of borate catalysed reactions compared with hydrogen peroxide and inorganic or organic peracids previously observed for the organic sulfides (D. M. Davies, M. E. Deary, K. Quill and R. A. Smith, Chem.–Eur. J., 2005, 11, 3552–3558) is also seen with substituted dimethyl aniline nucleophiles. This provides evidence that the pattern is not due to any latent electrophilic tendency of the organic sulfides and further supports dioxaborirane being the likely reactive intermediate, thus broadening the applicability of this catalytic system. Moreover, density functional theory calculations on our proposed mechanism involving dioxaborirane are consistent with the experimental results for these substrates. Results obtained at high concentrations of both borate and hydrogen peroxide require the inclusion the diperoxodiborate dianion in the kinetic analysis. A scheme detailing our current understanding of the borate–peroxide system is presented.

 Please wait while we load your content...
Please wait while we load your content...