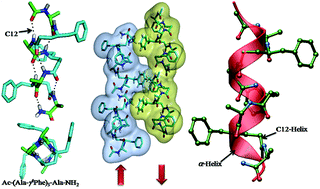
Numerous strategies have been developed to mimic the α-helical secondary structure using hybrid peptides containing non-natural amino acids. In contrast to the β- and α/β-hybrid peptides, very little is known about the folding patterns of hybrid peptides containing γ4-amino acids. Here we report the solid phase synthesis and crystallographic insight into the secondary structures formed by 1 : 1 alternating α/γ4-hybrid peptides. The crystal conformations suggest that heptapeptides P1, P2 and P3 adopted the 12-helix conformation with backward consecutive 1←4 H-bonds [C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O(i)⋯H–N (i + 3)]. In comparison with α-, β- and γ-peptides, the distinct projection of side-chains was observed along the helical cylinder. In contrast to the peptide containing stereochemically constrained α-amino acid Aib (P1), the peptide with complete proteinogenic side-chains (P3) displayed organized side chain–side chain interactions between the antiparallel helices in crystal packing. The analogy of the α/γ4-hybrid peptides with 310-helix, α-helix and β-peptide 12-helix suggests that the internal H-bonding pattern and macrodipole were analogous to the α- and β-peptide helices. In addition, helical parameters were found to be very similar to that of β-peptide 12-helices.
O(i)⋯H–N (i + 3)]. In comparison with α-, β- and γ-peptides, the distinct projection of side-chains was observed along the helical cylinder. In contrast to the peptide containing stereochemically constrained α-amino acid Aib (P1), the peptide with complete proteinogenic side-chains (P3) displayed organized side chain–side chain interactions between the antiparallel helices in crystal packing. The analogy of the α/γ4-hybrid peptides with 310-helix, α-helix and β-peptide 12-helix suggests that the internal H-bonding pattern and macrodipole were analogous to the α- and β-peptide helices. In addition, helical parameters were found to be very similar to that of β-peptide 12-helices.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O(i)⋯H–N (i + 3)]. In comparison with α-, β- and γ-
O(i)⋯H–N (i + 3)]. In comparison with α-, β- and γ-

 Please wait while we load your content...
Please wait while we load your content...