Enantioselective construction of 2,5-dihydropyrrole skeleton with quaternary stereogenic center via catalytic asymmetric 1,3-dipolar cycloaddition involving α-arylglycine esters†
Abstract
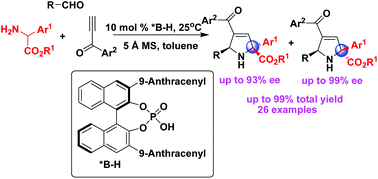
A catalytic asymmetric construction of synthetically and biologically important 2,5-dihydropyrrole scaffolds with concomitant creation of multiple chiral carbon centers including one quaternary stereogenic center in high yields (up to 99%) and excellent enantioselectivities (up to 99% ee) has been established via an organocatalytic 1,3-dipolar cycloaddition using α-arylglycine esters as azomethine precursors. Moreover, a detailed investigation has been performed on the catalytic asymmetric 1,3-dipolar cycloadditions of α-arylglycine ester-generated azomethine ylides with alkynes, providing an efficient way to simultaneously access both 2,5-dihydropyrrole diastereomers in good enantioselectivities.

 Please wait while we load your content...
Please wait while we load your content...