Low-temperature crystallization of anodized TiO2 nanotubes at the solid–gas interface and their photoelectrochemical properties†
Abstract
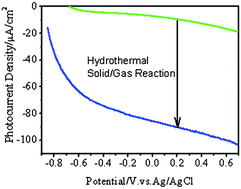
TiO2 nanotubular arrays formed by electrochemical anodization have attracted significant attention for photoelectrochemical applications that utilize solar energy. However, the as-anodized TiO2 nanotubes are amorphous, and need to be crystallized by high-temperature thermal annealing. Herein, we describe a low-temperature hydrothermal solid–gas route to crystallize TiO2 nanotubes. In this process, the as-anodized TiO2 hydroxo nanotubes are dehydrated to yield anatase phase via solid–gas interface reaction in an autoclave at a temperature of less than 180 °C. The solid–gas interface reaction alleviates the collapse of as-anodized TiO2 nanotubes during hydrothermal process efficiently. Compared with the common thermal annealing at the same temperature but at atmospheric pressure, the hydrothermal route improves the photocurrent density of TiO2 nanotubes by ∼10 times in KOH electrolyte. The duration of the hydrothermal reaction has a substantial effect on the photoelectrochemical properties of TiO2 nanotubes, which is ascribed to the synergetic effect between the crystallization and structural evolution. Electron donors can further suppress the charge recombination in the low-temperature crystallized TiO2 nanotubes and boost the photocurrent density by ∼120%.

 Please wait while we load your content...
Please wait while we load your content...