Fabrication of hollow metal oxide nanocrystals by etching cuprous oxide with metal(ii) ions: approach to the essential driving force†
Abstract
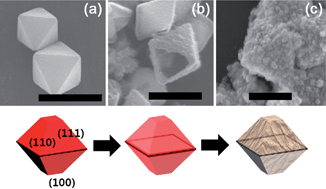
Hollow metal oxide nanocrystals were prepared by etching cuprous oxide with metal ions and were applied as photoelectrodes. As a hard template, polyvinylpyrrolidone stabilized cuprous oxide (PVP–Cu2O) and non-stabilized cuprous oxide (nPVP–Cu2O) were synthesized by a precipitation method. Hollow iron oxide and cobalt oxide nanocrystals with a truncated octahedral morphology were fabricated by an etching reaction with transition metal(II) ions (Fe2+ or Co2+). In the etching reaction process, a cationic exchange reaction occurs between the divalent metal ion and Cu+ due to the higher Lewis acidity. Facet selective etching of cuprous oxide has been observed during the ionic exchange reaction of Cu+ and O2− ions in PVP–Cu2O complexes with transition metal(II) ions (Fe2+ or Co2+) at the surface of a (110) facet. Amorphous states of hollow metal oxide products were annealed to form α-Fe2O3 (hematite) and Co3O4 and their crystal structure was examined with X-ray diffraction and HR-TEM. The optical absorption behavior of semiconductor nanocrystals was measured with UV-vis spectroscopy to define band gap energy. The hollow hematite structure has a 2.08 eV band gap and Co3O4 (Co(II,III) oxide) has a 1.80 eV indirect band gap. Using these hollow nanocrystals, a metal oxide monolayer film was fabricated with a secondary growth approach and was studied for its photocatalytic properties.

 Please wait while we load your content...
Please wait while we load your content...