Rapid atomic layer deposition of silica nanolaminates: synergistic catalysis of Lewis/Brønsted acid sites and interfacial interactions†
Abstract
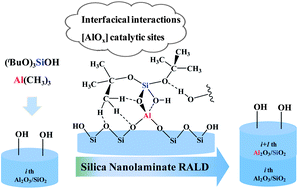
Rapid atomic layer deposition (RALD) has been applied to prepare various nanolaminates with repeated multilayer structures. The possible reaction pathways for RALD of the Al2O3/SiO2 nanolaminate using trimethylaluminum (TMA) and tris(tert-butoxy)silanol (TBS) are investigated by using density functional theory (DFT) calculations. The introduction of a Lewis-acid catalyst, TMA, can result in the formation of the catalytic site, which accelerates the propagation of the siloxane polymer. The rate-determining step of whole RALD is the elimination of isobutene of the tert-butoxy groups. The Brønsted acid site of [AlO4] can catalyze the elimination of isobutene. At the same time, the interfacial interactions, such as hydrogen bonding interactions between tert-butoxy groups and the surface, further catalyze the elimination of isobutene and accelerate SiO2 RALD reactions. The synergistic catalysis of Lewis/Brønsted acid sites and interfacial interactions may be applied in the RALD fabrication of other silica nanolaminates, such as HfO2/SiO2, ZrO2/SiO2, and TiO2/SiO2, in microelectronics, catalysis, energy storage, and conversion.

 Please wait while we load your content...
Please wait while we load your content...