Rapid two-step metallization through physicochemical conversion of Ag2O for printed “black” transparent conductive films
Abstract
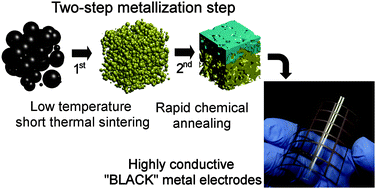
A rapid two-step metallization for fabrication of a “black” transparent conductive film on a flexible substrate for display applications is presented, using a mixture of silver oxide (Ag2O) and silver neodecanoate (C10H19AgO2), and its electrical conductivity and colour transition behaviours are investigated. Silver nanoparticles, which are physicochemically converted from silver oxide microparticles in the presence of silver neodecanoate in the course of the first metallization step at 150 °C for 10 min, are chemically annealed by immersing them in an acidic ferric chloride (FeCl3) solution at room temperature for 10 s. During this second metallization step, silver nanoparticles are found to be tightly packed through Ostwald ripening, which eventually leads to the dramatic enhancement of electrical conductivity by six orders of magnitude from 1.33 S m−1 to 1.0 × 107 S m−1, which corresponds to 15.9% of the electrical conductivity of bulk silver. In addition to the enhancement of electrical conductivity, the silver chloride (AgCl) layer formed on the surface of the silver layer due to ferric ions (Fe3+) enhances the blackness of the transparent conductive film by a factor of 1.69, from 36.29 B to 61.51 B. The sheet resistance and optical transparency of a roll-to-roll printed black transparent conductive film for a touch screen panel are found to be as low as 0.9 Ω □−1 and 81%, respectively, after conducting the proposed two-step metallization.

 Please wait while we load your content...
Please wait while we load your content...