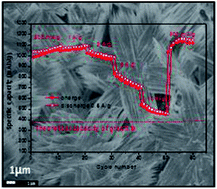
A novel hierarchical star-like Co3O4 was successfully synthesized from self-assembled hierarchical Co(OH)F precursors via a facile hydrothermal method and subsequent annealing in air. The morphological evolution process of the Co(OH)F precursors was investigated by examining the different reaction times during synthesis. First, hexagonal plates are formed, and then nanodiscs grow on the surface of the plates. Subsequently, dissolution and regrowth of Co(OH)F occur to form the star-like hierarchical structures. Co3O4 obtained from thermal decomposition of the Co(OH)F precursor in air at 350 °C exhibited high reversible capacity as an anode material in lithium ion batteries. The specific charge capacity of 1036 mA h g−1 was obtained in the first cycle at a current density of 50 mA g−1, and after 100 cycles, the capacity retention was nearly 100%. When the current density was increased to 500 mA g−1 and 2 A g−1, the capacities were 995 and 641 mA h g−1, respectively, after 100 cycles. In addition, a capacity of 460 mA h g−1 was recorded at a current density of 10 A g−1 in the rate capability test. The excellent electrochemical performance of the Co3O4 electrodes can be attributed to the porous interconnected hierarchical nanostructures, which protect the small particles from agglomeration and buffer the volume change during the discharge–charge process.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...