Eight new energetic salts based on 1-methoxy-5-nitroiminotetrazole, which was obtained by nitration of 1-methoxy-5-aminotetrazole, were synthesized. Guanidinium (5), aminoguanidinium (6), diaminoguanidinium (7), triaminoguanidinium (8), carbohydrazidinium (9), 3-amino-1,2,4-triazolium (10), 4-amino-1,2,4-triazolium (11), and 3,5-diamino-1,2,4-triazolium (12) salts were characterized by vibrational spectroscopy (IR, Raman), multinuclear spectroscopy (1H, 13C, 15N), elemental analysis, and single crystal X-ray diffraction analysis. Salts 5·1/3H2O and 7–9 crystallize in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , whereas compounds 6 and 10 crystallize in the monoclinic space groups C2/c and P2(1)/n, respectively. Compound 11 is in orthorhombic group P2(1)2(1)2(1). In addition, the heats of formation (ΔHf), and detonation properties (pressure and velocity) were calculated using Gaussian 03 and EXPLO5 programs, respectively. Thermal stabilities were obtained by DSC measurements and the sensitivities toward impact and friction were determined by BAM methods.
, whereas compounds 6 and 10 crystallize in the monoclinic space groups C2/c and P2(1)/n, respectively. Compound 11 is in orthorhombic group P2(1)2(1)2(1). In addition, the heats of formation (ΔHf), and detonation properties (pressure and velocity) were calculated using Gaussian 03 and EXPLO5 programs, respectively. Thermal stabilities were obtained by DSC measurements and the sensitivities toward impact and friction were determined by BAM methods.
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , whereas compounds 6 and 10 crystallize in the monoclinic space groups C2/c and P2(1)/n, respectively. Compound 11 is in orthorhombic group P2(1)2(1)2(1). In addition, the heats of formation (ΔHf), and detonation properties (pressure and velocity) were calculated using Gaussian 03 and EXPLO5 programs, respectively. Thermal stabilities were obtained by
, whereas compounds 6 and 10 crystallize in the monoclinic space groups C2/c and P2(1)/n, respectively. Compound 11 is in orthorhombic group P2(1)2(1)2(1). In addition, the heats of formation (ΔHf), and detonation properties (pressure and velocity) were calculated using Gaussian 03 and EXPLO5 programs, respectively. Thermal stabilities were obtained by 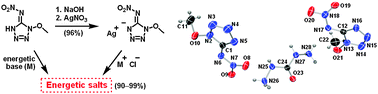

 Please wait while we load your content...
Please wait while we load your content...