Heads vs. tails: a double-sided study of the influence of substituents on the glass-forming ability and stability of aminotriazine molecular glasses†
Abstract
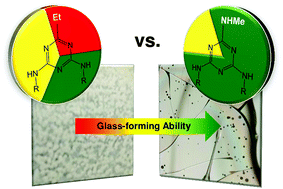
Mexylaminotriazine derivatives are known to spontaneously form long-lived glassy phases. The role played by various structural elements in their glass formation has been studied, but the effect of substituting both arylamino substituents remains largely unknown. A library of 4,6-bis(arylamino)- or 4,6-bis(alkylamino)-1,3,5-triazine derivatives with a methylamino or ethyl substituent in the 2-position were synthesized, and their glass-forming properties were studied. While the 3,5-disubstituted aryl motif proved to be the best among those studied for promoting glass formation, glass-forming ability and stability were found to be in large part influenced by the “headgroup” at the 2-position of the triazine ring, with dramatic differences in glass-forming behavior observed from one headgroup to the other.

 Please wait while we load your content...
Please wait while we load your content...