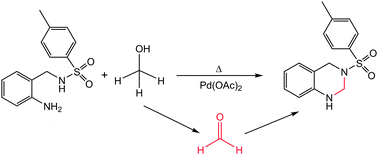
Both experimental and computational studies were undertaken to elucidate the formation process of 3-tosyl-1,2,3,4-tetrahydroquinazoline from methanolic mother liquors of Pd(LBS)·3H2O, where LBS is the dianionic form of the imine ligand N-{2-[(8-hydroxyquinolin-2-yl)methyleneamino]benzyl}-4-methylbenzenesulfonamide. Experimental studies have shown that the tetrahydroquinazoline is obtained by condensation of 2-tosylaminomethylaniline and formaldehyde, which come from the acid-catalyzed hydrolysis of the imine ligand LBS and metal-mediated aerobic oxidation of methanol, respectively. Computational studies have revealed relevant intermediates and key steps in the reaction pathway.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...