Natural mineral pyrite and analytical application thereof in precipitation titrations in non-aqueous solvents
Abstract
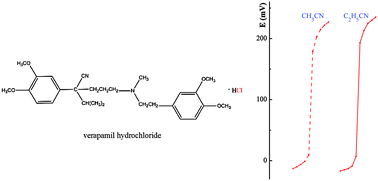
In this study, a potentiometric sensor, based on the natural mineral pyrite as an electroactive material, is used for a simple and fast determination of different halide and thiocyanate ions such as hydroxylammonium chloride, potassium iodide, tetrabutylammonium bromide, and ammonium thiocyanate, in addition to titrations of some biologically important systems (choline chloride, cetyltrimethylammonium bromide), which include verapamil hydrochloride as well as its determination in pharmaceutical formulation. Verapamil is a calcium channel blocker used in the management of angina, arrhythmia and hypertension. Halide and thiocyanate ions can be determined by a precipitation titration with silver nitrate as the titrant in non-aqueous solutions, and the end-point can be evaluated by a potentiometric method, in which a pyrite electrode is used as the indicator electrode. The sensor shows a stable, near Nernstian response, over the concentration range 1.0 × 10−4–1.0 × 10−1 M of standard silver ion solutions at 25 °C with a cationic slope of 63.6 and 59.3 mV per decade in acetonitrile and propionitrile, respectively. The wide linear range, fast response time (∼15 s), reproducibility, a long service life without considerable divergence in potentials are characteristics of the proposed sensor for precipitation determination in acetonitrile and propionitrile. Potentiometric determination of 10–80 mg of the investigated substances shows an average recovery of 98.7% and a mean standard deviation of 0.6%.

 Please wait while we load your content...
Please wait while we load your content...