Synthesis of analogues of linckoside B, a new neuritogenic steroid glycoside
Abstract
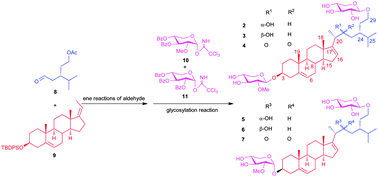
A facile synthetic approach toward six designed analogues (2–7) of linckoside B, a new neuritogenic steroid glycoside isolated from the Okinawan starfish Linckia laevigata, has been developed. The key steroid aglycon was achieved by a dimethylaluminum chloride-mediated ‘ene’ reaction. A versatile strategy was employed for the construction of glycosidic bonds through Schmidt's procedure using a glycosyl trichloroacetimidate donor.

 Please wait while we load your content...
Please wait while we load your content...