Synthesis and in vitro cytotoxicity of deoxyadenosine–bile acid conjugates linked with 1,2,3-triazole
Abstract
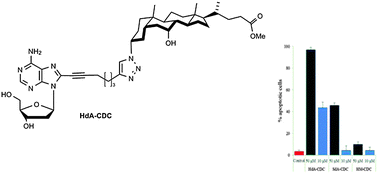
We report herein the synthesis and biological evaluation of novel deoxynucleoside–bile acid conjugates linked through a 1,2,3-triazole ring. The conjugates were synthesized via Cu(I) mediated 1,3-dipolar cycloaddition reaction (‘click’ chemistry) of 3-azidobile acid derivatives and terminal alkyne moieties linked to the C-8 position of deoxyadenosine. All novel molecules were evaluated in vitro for their anti-proliferative activity against four human cell lines (i.e., leukemic T Jurkat and K562; colon carcinoma HCT116; and ovarian cancer A2780) and their cytotoxicity toward human fibroblast cells. Several conjugates exhibited strong anti-proliferative activity against human leukemia T cells. The best cytotoxicity was observed for HdA-CDC on both leukemia cell lines with IC50 up to 8.51 μM. The apoptotic activity of several conjugates was also established.
- This article is part of the themed collection: In honour of Bernard Meunier

 Please wait while we load your content...
Please wait while we load your content...