Kinetics and mechanism of hexachloroiridate(iv) oxidation of tellurium(iv) in aqueous solutions
Abstract
The kinetics of oxidation of tellurium(IV) by [IrCl6]2− in aqueous perchlorate solutions at a constant ionic strength of 0.1 mol dm−3 have been investigated spectrophotometrically. The results showed a first-order dependence in [IrCl6]2−, fractional-first-order kinetics with respect to tellurium(IV) concentration and second-order overall kinetics. The increase in the [H+] was found to be accompanied by a decrease in the rate constants, i.e., the oxidation reaction was acid-inhibited. Kinetic evidence for the formation of a 1 : 1 intermediate binuclear complex between the two reactants has been obtained. The ionization constant of tellurium(IV) has been evaluated and found to be 1.69 × 10−3 dm3 mol−1 at 25 °C. The kinetic parameters have been calculated and a tentative reaction mechanism consistent with the kinetic data is suggested.
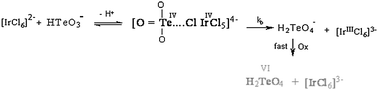

 Please wait while we load your content...
Please wait while we load your content...