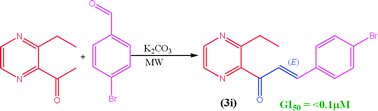
Two series of novel (E)-3-aryl-1-(3-alkyl-2-pyrazinyl)-2-propenones (3a–3f and 3g–3l) have been synthesized by the Claisen–Schmidt condensation reaction between 2-acetyl-3-alkyl pyrazine (0.005 mol, 1a–1b) and para-substituted aromatic aldehydes (0.005 mol, 2a–2f) under solvent-free K2CO3 solid supported microwave environment, with 90–95% yield. The structures were confirmed by FTIR, 1H NMR, 13C NMR, LCMS (Q-TOF) and elemental analysis. The mean surface roughness values (Ra) of 10.59 and 10.87 nm for 3c and 3i, respectively, were measured with AFM. Compounds were tested for their in vitro anticancer activity against the MCF-7 cell line using sulforhodamine B (SRB) assay protocols to estimate cell growth and compared with adriamycin. Compound 3i containing p-Br on the phenyl ring expressed promising anticancer activity (GI50 ≤ 0.1 μM). The cytotoxicity (LC50) was found in the range of 86 to >100 μM as compared to that of the standard (LC50 = 89 μM). Also, compounds were screened for in vitro antibacterial and antifungal activities, and the most prominent effects were observed with 3a, 3c, 3d and 3e against gram-positive and gram-negative strains. Compounds 3a–3l showed 30–66% antioxidant activities, determined by 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical method. The viscosity as a transport property was studied for their entire composition range at 298.15 K. Data were regressed against concentration for limiting viscosity and noted as 3h > 3g > 3i = 3l > 3k > 3a > 3j > 3f > 3e > 3c > 3d > 3b.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...