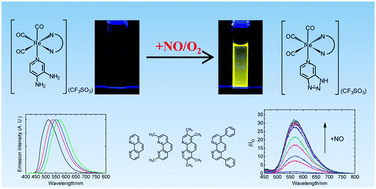
A series of rhenium(I) polypyridine complexes functionalized with a diaminoaromatic moiety has been developed as phosphorescent sensors for nitric oxide (NO). The diamine complexes [Re(N⁁N)(CO)3(py-diamine)](CF3SO3) [py-diamine = 3,4-diaminopyridine; N⁁N = 1,10-phenanthroline (phen) (1a), 2,9-dimethyl-1,10-phenanthroline (Me2-phen) (2a), 3,4,7,8-tetramethyl-1,10-phenanthroline (Me4-phen) (3a), 4,7-diphenyl-1,10-phenanthroline (Ph2-phen) (4a)] were synthesized and characterized. These complexes were only weakly emissive due to the diaminoaromatic moiety that quenches the 3MLCT [dπ(Re) → π*(N⁁N)] emission by photoinduced electron transfer (PET). However, in the presence of NO, these diamine complexes were converted to the triazole derivatives [Re(N⁁N)(CO)3(py-triazole)](CF3SO3) [py-triazole = 1H-1,2,3-triazolo[4,5,c]pyridine; N⁁N = phen (1b), Me2-phen (2b), Me4-phen (3b), Ph2-phen (4b)], which revealed intense emission upon excitation. The emission of the complexes was independent to pH under neutral and basic conditions (pH > 6). The cytotoxicity and cellular uptake properties of these complexes were studied by the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) assay and ICP-MS, respectively. The potential application of these complexes as intracellular NO sensors was also investigated.

 Please wait while we load your content...
Please wait while we load your content...