Synthesis, electronic properties and packing modes of conjugated systems based on 2,5-di(cyanovinyl)furan or thiophene and imino-perfluorophenyl moieties†‡
Abstract
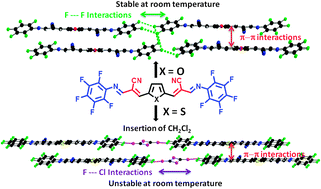
The synthesis, the electronic properties and the solid state arrangements of conjugated systems associating

 Please wait while we load your content...
Please wait while we load your content...