Cleaving bonds in CH3OSO2CF3 with [1,2,4-(Me3C)3C5H2]2CeH; an experimental and computational study†‡
Abstract
The reaction at 20 °C of the metallocenelanthanide 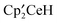 , and excess
, and excess 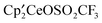 ,
, 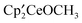 ,
, 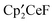 and the bimetallic complex
and the bimetallic complex 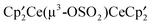 . The
. The 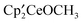 ,
, 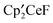 , and
, and 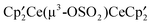 react with excess CH3OSO2CF3 to form
react with excess CH3OSO2CF3 to form 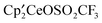 , CH3OCH3, CH3F, and (CH3O)2SO, respectively, at 20 °C. Thus, the net reaction is
, CH3OCH3, CH3F, and (CH3O)2SO, respectively, at 20 °C. Thus, the net reaction is 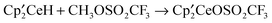 but the pathway is not a direct
but the pathway is not a direct 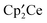 fragments, is unique in organometallic chemistry. The
fragments, is unique in organometallic chemistry. The 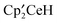 show that the CH-bond activation and direct CH3 group transfer have similar
show that the CH-bond activation and direct CH3 group transfer have similar 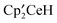 with CH3OSO2CH3, where CH-bond
with CH3OSO2CH3, where CH-bond 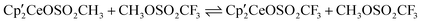 . This contrasts, perhaps, with conventional wisdom that overemphasizes the effect of the electron-withdrawing ability of the CF3 group on the chemical and physical properties of
. This contrasts, perhaps, with conventional wisdom that overemphasizes the effect of the electron-withdrawing ability of the CF3 group on the chemical and physical properties of
![Graphical abstract: Cleaving bonds in CH3OSO2CF3 with [1,2,4-(Me3C)3C5H2]2CeH; an experimental and computational study](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C2NJ40624A&imageInfo.ImageIdentifier.Year=2013)
- This article is part of the themed collection: “All Aboard” – contributions from NJC Board members

 Please wait while we load your content...
Please wait while we load your content...