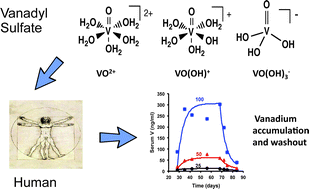
Vanadium, abbreviated V, is an early transition metal that readily forms coordination complexes with a variety of biological products such as proteins, metabolites, membranes and other structures. The formation of coordination complexes stabilizes metal ions, which in turn impacts the biodistribution of the metal. To understand the biodistribution of V, V in oxidation state IV in the form of vanadyl sulfate (25, 50, 100 mg V daily) was given orally for 6 weeks to 16 persons with type 2 diabetes. Elemental V was determined using Graphite Furnas Atomic Absorption Spectrometry against known concentrations of V in serum, blood or urine. Peak serum V levels were 15.4 ± 6.5, 81.7 ± 40 and 319 ± 268 ng ml−1 respectively, and mean peak serum V was positively correlated with dose administered (r = 0.992, p = 0.079), although large inter-individual variability was found. Total serum V concentration distribution fit a one compartment open model with a first order rate constant for excretion with mean half times of 4.7 ± 1.6 days and 4.6 ± 2.5 days for the 50 and 100 mg V dose groups respectively. At steady state, 24 hour urinary V output was 0.18 ± 0.24 and 0.97 ± 0.84 mg in the 50 and 100 mg V groups respectively, consistent with absorption of 1 percent or less of the administered dose. Peak V in blood and serum were positively correlated (r = 0.971, p < 0.0005). The serum to blood V ratio for the patients receiving 100 mg V was 1.7 ± 0.45. Regression analysis showed that glycohemoglobin was a negative predictor of the natural log(ln) peak serum V (R2 = 0.40, p = 0.009) and a positive predictor of the euglycemic–hyperinsulinemic clamp results at high insulin values (R2 = 0.39, p = 0.010). Insulin sensitivity measured by euglycemic–hyperinsulinemic clamp was not significantly correlated with ln peak serum V. Globulin and glycohemoglobin levels taken together were negative predictors of fasting blood glucose (R2 = 0.49, p = 0.013). Although V accumulation in serum was dose-dependent, no correlation between total serum V concentration and the insulin-like response was found in this first attempt to correlate anti-diabetic activity with total serum V. This study suggests that V pools other than total serum V are likely related to the insulin-like effect of this metal. These results, obtained in diabetic patients, document the need for consideration of the coordination chemistry of metabolites and proteins with vanadium in anti-diabetic vanadium complexes.

 Please wait while we load your content...
Please wait while we load your content...