The A-CD analogue of 16β,17α-estriol is a potent and highly selective estrogen receptor β agonist†
Abstract
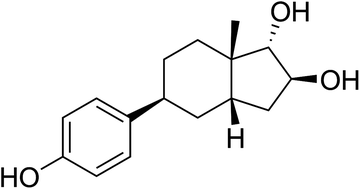
Selective estrogen receptor β (ERβ) agonists display neuroprotective properties in animal models and hold promise in the treatment of neurodegenerative diseases. In our quest to design, synthesize and evaluate potent and safe ERβ agonists, we focused on making an analogue of 16β,17α-estriol (16,17-epiestriol), a potent and one of the most ERβ selective endogenous estrogens reported. Herein we disclose the synthesis and in vitro evaluation of an analogue based on the recently introduced A-CD scaffold. A 14-step synthesis based on the Hajos–Parrish ketone resulted in the discovery of (1S,2S,3aS,5S,7aS)-5-(4-hydroxyphenyl)-7a-methyloctahydro-1H-indene-1,2-diol (15). This A-CD analogue of 16β,17α-estriol is a highly selective (500-fold) ERβ full agonist over ERα with a pEC50 of 7.7 at ERβ. Molecular modelling suggests that 15 turns around in the ligand-binding domain compared to estriol, thus the 7a-methyl occupies the α-face, which might explain the high selectivity.
 Please wait while we load your content...
Please wait while we load your content...