Mechanistic insight into inhibition of two-component system signaling†
Abstract
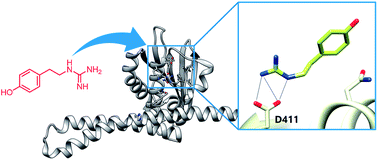
Two-component signal transduction systems (TCSs) are commonly used by bacteria to couple environmental stimuli to adaptive responses. Targeting the highly conserved kinase domain in these systems represents a promising strategy for the design of a broad-spectrum antibiotic; however, development of such compounds has been marred by an incomplete understanding of the conserved binding features within the active site that could be exploited in molecule design. Consequently, a large percentage of the available TCS inhibitors demonstrate poor target specificity and act via multiple mechanisms, with aggregation of the kinase being the most notable. In order to elucidate the mode of action of some of these compounds, molecular modeling was employed to dock a suite of molecules into the ATP-binding domain of several histidine kinases. This effort revealed a key structural feature of the domain that is likely interacting with several known inhibitors and is also highly conserved. Furthermore, generation of several simplified scaffolds derived from a reported inhibitor and characterization of these compounds using activity assays, protein aggregation studies and saturation transfer differential (STD) NMR suggests that targeting of this protein feature may provide a basis for the design of ATP-competitive compounds.

 Please wait while we load your content...
Please wait while we load your content...