Novel benzothiazole derivatives with a broad antifungal spectrum: design, synthesis and structure–activity relationships†
Abstract
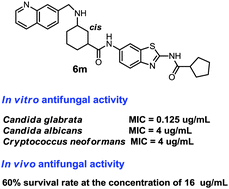
N-Myristoyltransferase (NMT) is a new target for the development of novel antifungal agents. The benzothiazole derivative FR1335 is a highly potent NMT inhibitor with fungicidal activity. However, FTR1335 was only active against Candida albicans and its antifungal spectrum and solubility remained to be improved. Thus, a series of benzothiazole derivatives were designed and synthesized by modifying the scaffold and side chain of FTR1335. Interestingly, the antifungal spectrum of the target compounds was significantly expanded and also new structure–activity relationship information was obtained. Particularly, compound 6m showed good inhibitory activity against a wide range of fungal pathogens including systemic fungus and dermatophytes. Its antifungal activity against Cryptococcus neoformans and Candida glabrata was higher than that of fluconazole. Moreover, compound 6m also exhibited in vivo antifungal activity in a Caenorhabditis elegans-Candida albicans infection model, which represents a promising lead for further optimization.
 Please wait while we load your content...
Please wait while we load your content...