Dual mode of action of phenyl-pyrazole-phenyl (6-5-6 system)-based PPI inhibitors: alpha-helix backbone versus alpha-helix binding epitope†
Abstract
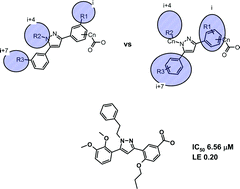
A new class of alpha-helix mimetics, based on the phenyl-pyrazole-phenyl (6-5-6) system, has been designed and synthesized. The ability of the new compounds to inhibit PPIs was confirmed using an MDM2-p53 binding assay. The library, containing completely new compounds, revealed an excellent hit rate of 15%, had satisfactory physicochemical properties (~38% soluble compounds), and the ligand efficiency of the best compound was 0.21 (0.22 for nutlin-3 in the same assay). Dual mode of action of these inhibitors was suggested based on computer modeling: depending on the nature of their substituents they could act as either an alpha-helix backbone mimetic or an alpha-helix binding epitope mimetic.
 Please wait while we load your content...
Please wait while we load your content...