Trifluoromethyl derivatives of canonical nucleosides: synthesis and bioactivity studies†
Abstract
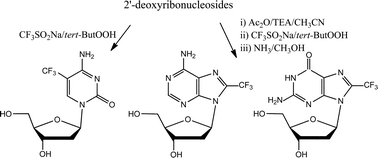
The use of the system CF3SO2Na/tert-butyl-hydroperoxide (tert-ButOOH), recently reported for the efficient trifluoromethylation of a variety of heterocyclic aromatic compounds, has been here profitably exploited for the synthesis of 5-CF3-2′-deoxycytidine, 8-CF3-2′-deoxyadenosine, 8-CF3-2′-deoxyguanosine and 8-CF3-inosine, regioselectively obtained in good to acceptable yields following a very simple protocol. The bioactivity of these modified nucleosides, and particularly of the novel 8-CF3-2′-deoxyguanosine and 8-CF3-inosine, has been evaluated on a panel of tumour and non-tumour cell lines in preliminary in vitro cytotoxicity assays.

 Please wait while we load your content...
Please wait while we load your content...