A critical assessment of modeling safety-related drug attrition
Abstract
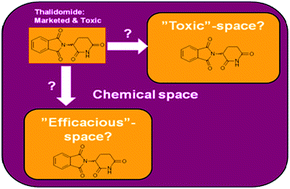
This paper challenges the general desire to find simple rules and guidelines to reduce attrition due to toxicity and clinical safety. We present an analysis of 150 AstraZeneca development compounds and evaluate some of the published guidelines and their ability to identify compounds with safety liabilities. Interestingly, none of the current guidelines were able to discriminate compounds that successfully reached Phase II. The analysis was extended to recently approved

 Please wait while we load your content...
Please wait while we load your content...