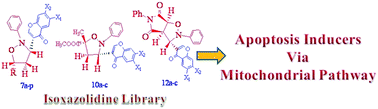
Synthesis and cytotoxicity evaluation of regioisomeric substituted N-phenyl-3′-(chrom-4-one-3-yl)-isoxazolidines: induction of apoptosis through a mitochondrial-dependent pathway†
Abstract
Regio- and stereoselective 1,3-dipolar cycloadditions of C-(chrom-4-one-3-yl)-N-phenylnitrones (5) with different mono-substituted, disubstituted and cyclic dipolarophiles were carried out to obtain substituted N-phenyl-3′-(chrom-4-one-3-yl)-isoxazolidines (7a–p, 10a–c and 12a–c). Some of the obtained isoxazolidines display significant cytotoxicity against several human cancer cell lines. The preliminary bioassay results revealed that compound 10c showed higher cytotoxicity than
 Please wait while we load your content...
Please wait while we load your content...