Combination of biotransformation by P450 BM3 mutants with on-line post-column bioaffinity and mass spectrometric profiling as a novel strategy to diversify and characterize p38α kinase inhibitors†
Abstract
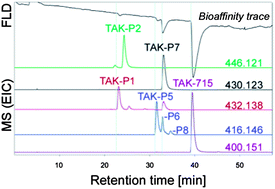
Mutants of bacterial cytochrome P450 BM3 from Bacillus megaterium have gained increasing interest to support drug metabolism studies by producing human relevant biotransformation products and/or to support drug discovery by producing libraries of new chemical entities based on initial lead compounds. In this study, a library of 33 P450 BM3 mutants was used to diversify TAK-715, a representative (highly lipophilic) inhibitor of p38α mitogen-activated protein kinase (p38α). To simultaneously determine the individual bioaffinity and identity of the different products, an analytical high-resolution screening approach was used, based on post-column on-line bioaffinity profiling with parallel mass spectrometric identification. The screening of the product mixtures produced demonstrated that introducing mutations in the active site of the P450 BM3 resulted in different product profiles. Several P450 BM3 mutants were mimicking the metabolic profile obtained by human liver microsomes. The major biotransformation products of TAK-715 could be produced with the most active P450 BM3 mutant in sufficient amounts to enable further structure elucidation by 1H-NMR and quantification of their p38α affinity.
 Please wait while we load your content...
Please wait while we load your content...