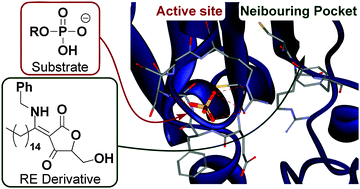
RE derivatives, which are cell-permeable and non-electrophilic dual-specificity protein phosphatase inhibitors developed in our laboratory, inhibit CDC25A/B non-competitively, as determined by means of kinetic experiments. To identify the binding site of RE derivatives, we designed and synthesized the new probe molecule RE142, having a Michael acceptor functionality for covalent bond formation with the enzyme, a biotin tag to enable enrichment of probe-bound peptide(s), and a chemically cleavable linker to facilitate release of probe-bound peptides from avidin beads. LC-MS analysis indicated that RE142 binds to one of the residues Cys384-Tyr386 of CDC25A, within a pocket adjacent to the catalytic site.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...