The novel equisetin-like compound, TA-289, causes aberrant mitochondrial morphology which is independent of the production of reactive oxygen species in Saccharomyces cerevisiae†
Abstract
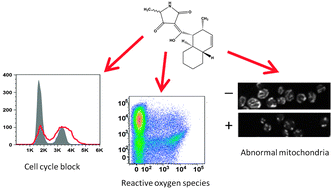
Tetramic acids constitute a large class of natural products isolated from a variety of different fungal and bacterial species. While the presence of the distinctive 2,4-pyrrolidinedione ring system defines this class of compounds, these compounds are widely diverse both structurally and in the biological activities that they display. Equisetin-like compounds are tetramic acids that have been shown to be growth inhibitory towards bacteria, fungi, yeasts and mammalian cell lines; however, the mechanisms inhibiting prokaryotic and eukaryotic cell growth have not been fully explained. Here we report the isolation and biological characterisation of a novel equisetin-like tetramic acid named tetramic acid-289 (TA-289) produced by a Fusarium sp. fungus. This compound displayed pH- and carbon source-dependent cytotoxic effects in Saccharomyces cerevisiae and caused an irreversible cell cycle block via a microtubule independent mechanism. To fully elucidate a mechanism, we used an unbiased approach employing chemogenomic profiling of the yeast deletion library and demonstrated that TA-289 hypersensitive deletion strains are also sensitive to oxidants, respiratory

 Please wait while we load your content...
Please wait while we load your content...