Solution conformational features and interfacial properties of an intrinsically disordered peptide coupled to alkyl chains: a new class of peptide amphiphiles†‡
Abstract
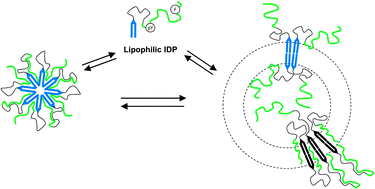
Owing to the large panel of biological functions of
* Corresponding authors
a
Dipartimento di Farmacia and CIRPeB, Università degli Studi Federico II di Napoli, Via Mezzocannone, 16 80134 Naples, Italy
E-mail:
filomena.rossi@unina.it
Fax: +39 0812534574
Tel: +39 0812536682
b Istituto di Biostrutture e Bioimmagini (IBB) CNR, Via Mezzocannone, 16 80134 Naples, Italy
c CNRS, Aix-Marseille Université, Enzymologie Interfaciale et Physiologie de la Lipolyse UMR 7282, Marseille, France
d Architecture et Fonction des Macromolécules Biologiques, UMR 7257, CNRS and Aix-Marseille Université, Marseille, France
Owing to the large panel of biological functions of

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
A. Accardo, M. Leone, D. Tesauro, R. Aufiero, A. Bénarouche, J. Cavalier, S. Longhi, F. Carriere and F. Rossi, Mol. BioSyst., 2013, 9, 1401 DOI: 10.1039/C3MB25507G
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
