A novel mechanism for antiglycative action of limonene through stabilization of protein conformation
Abstract
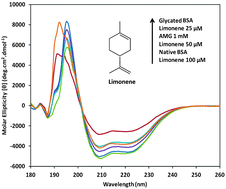
Inhibition of protein glycation is known to ameliorate secondary complications in diabetes. In the present study antiglycative properties of limonene, a natural product, were evaluated using BSA as a model protein. AMG (aminoguanidine) was used as a positive control. Measurement of total AGEs (Advanced Glycation End-products) and specific AGEs revealed that limonene could inhibit protein glycation to the extent of 56.3% and 75.1% respectively at 50 μM concentration as against 54.4% and 82.2% by AMG at 1 mM. Congo red binding and CD (Circular Dichroism) analysis revealed inhibition of α-helix to β-sheet transition wherein 18.5% β-sheet structures were observed in glycated BSA (bovine serum albumin) as against 4.9% with limonene. Glycation of protein in the presence of urea was enhanced by 18%, while in the presence of limonene it was reduced by 23% revealing the stabilizing effect of limonene. Electrophoretic mobility was similar to the normal control and a zeta potential value of −12.1 mV as against −15.1 mV in diabetic control was observed. Inhibition of glycation in limonene treated samples was confirmed through LC-MS analysis wherein AGEs such as pentosidine, CML (Nε-(carboxymethyl)lysine), CEL (Nε-(carboxyethyl)lysine), MOLD (methylglyoxal-lysine dimer) and imidazolone observed in glycated samples were absent in limonene treated samples. PatchDock studies revealed that limonene could bind to the major glycation sites IB, IIA and IIB sub domains and AMG to the IIIA sub domain. Thus limonene is a potent protein glycation inhibitor that prevents protein glycation through a novel mechanism of stabilization of protein structure through hydrophobic interactions.

 Please wait while we load your content...
Please wait while we load your content...