Ion channel recordings on an injection-molded polymer chip†
Abstract
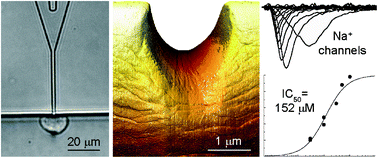
In this paper, we demonstrate recordings of the ion channel activity across the cell membrane in a biological cell by employing the so-called patch clamping technique on an injection-molded polymer microfluidic device. The findings will allow direct recordings of ion channel activity to be made using the cheapest materials and production platform to date and with the potential for very high throughput. The employment of cornered apertures for cell capture allowed the fabrication of devices without through holes and via a scheme comprising master origination by dry etching in a silicon substrate, electroplating in nickel and injection molding of the final part. The most critical device parameters were identified as the length of the patching capillary and the very low surface roughness on the inside of the capillary. The cross-sectional shape of the orifice was found to be less critical, as both rectangular and semicircular profiles seemed to have almost the same ability to form tight seals with cells with negligible leak currents. The devices were functionally tested using human embryonic kidney cells expressing voltage-gated sodium channels (Nav1.7) and benchmarked against a commercial state-of-the-art system for automated ion channel recordings. These experiments considered current–voltage (IV) relationships for activation and inactivation of the Nav1.7 channels and their sensitivity to a local anesthetic, lidocaine. Both IVs and lidocaine dose–response curves obtained from the injection-molded polymer device were in good agreement with data obtained from the commercial system.

 Please wait while we load your content...
Please wait while we load your content...