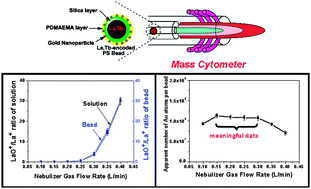
We describe the preparation of La, Tb-encoded-AuNP-coated PS microbeads via the combination of two-stage dispersion polymerization and post-functionalization of the particle surface. The introduction of La and Tb ions into the beads was achieved by the addition of LnCl3 and TbCl3 salts along with acrylic acid in the second the stage of dispersion polymerization. After the coating the surface of the beads with a silica layer containing methacrylate functional groups, a poly(N,N-dimethylaminoethyl methacrylate) (PDMAEMA) layer was introduced by free radical polymerization. Subsequently, the PDMAEMA layer hosted the formation of gold nanoparticles on the surface of the beads. The formation of gold nanoparticles (AuNPs) on the surface of the beads was confirmed by dark-field transmission electron microscopy (TEM) combined with energy-dispersive X-ray spectroscopy (EDX) and mass cytometry. Release experiments showed a negligible loss of Au ions or gold nanoparticles in 1.0% HCl aqueous solution over a two week period. The La, Tb-encoded-AuNP-coated PS beads were used to investigate the influence of plasma conditions on the thermolytic breakdown, diffusion, and matrix effects in the mass cytometer having two “thermometer” elements La (imbedded in the bulk of the bead) and Au (located on the bead surface). These experiments defined optimum conditions in terms of uniform ionization of the Au atoms present in the AuNPs. They also showed that neither the carbon-rich PS matrix nor the thin silica coating of the 1.6 μm diameter PS particles influenced the ability of the mass cytometer to determine La and Tb ion content quantitatively.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...