Redox reactions in Prussian blue containing paint layers as a result of light exposure†
Abstract
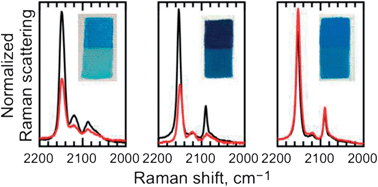
Prussian blue, a mixed valence pigment, typically KFeIII[FeII(CN)6]·xH2O, was the most widely used blue artistic pigment from ca. 1720 to the 1970's but, unfortunately, its paint layers, especially when used in conjunction with a white pigment, tend to fade or turn green upon extended exposure to light. In order to identify the mechanism underlying these changes, paint layers have been prepared with differing amounts of these white pigments and subjected to accelerated light exposure fading. The resulting unfaded and faded paint layers as well as both the Berlin white pigment, Fe2II[FeII(CN)6], and the partially oxidized Berlin green pigment, {KFeIII[FeII(CN)6]}x{FeIII[FeIII(CN)6]}1−x, have been characterized by Raman and iron-57 Mössbauer

 Please wait while we load your content...
Please wait while we load your content...