Cell mediated contraction in 3D cell-matrix constructs leads to spatially regulated osteogenic differentiation
Abstract
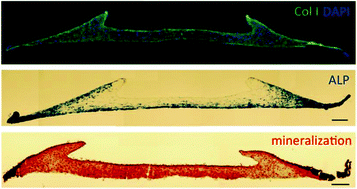
During embryonic development, morphogenetic processes give rise to a variety of shapes and patterns that lead to functional tissues and organs. While the impact of chemical signals on these processes is widely studied, the role of physical cues is less understood. The aim of this study was to test the hypothesis that the interplay of cell mediated contraction and mechanical boundary conditions alone can result in spatially regulated differentiation in simple 3D constructs. An experimental model consisting of a 3D cell–gel construct and a finite element (FE) model were used to study the effect of cellular traction exerted by mesenchymal stem cells (MSCs) on an initially homogeneous

 Please wait while we load your content...
Please wait while we load your content...