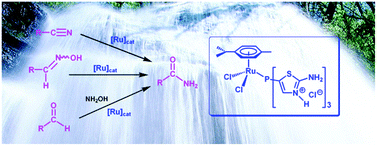
A series of water-soluble N-protonated thiazolyl-phosphine hydrochloride salts have been synthesized and coordinated to the ruthenium(II) fragment [RuCl2(η6-p-cymene)]. The resulting complexes were evaluated as potential catalysts for the selective hydration of nitriles to primary amides in environmentally friendly aqueous medium. The best results in terms of activity were achieved when tris(5-(2-aminothiazolyl))phosphine trihydrochloride was used as ligand. Using the Ru(II) complex 9 derived from this salt (3 mol%), the catalytic reactions proceeded cleanly in pure water at 100 °C without the assistance of any additive, affording the desired amides in high yields (>78%) after short reaction periods (0.5–7 h). The process was operative with both aromatic, heteroaromatic, α,β-unsaturated and aliphatic nitriles, and tolerated several functional groups. The utility of 9 in promoting the formation of primary amides in water by catalytic rearrangement of aldoximes and direct coupling of aldehydes with NH2OH·HCl has also been demonstrated.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...