H2O2-mediated oxidative formation of amides from aromatic amines and 1,3-diketones as acylation agents via C–C bond cleavage at room temperature in water under metal-free conditions†
Abstract
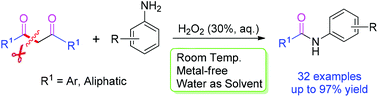
1,3-Diketones, as novel acylation agents, reacted with aromatic amines promoted by commercially available H2O2 (30% aq.) as the sole oxidant at room temperature under metal-free conditions in water, leading to a novel and rapid amide bond formation strategy. The reported method is high-yielding, simple and mild, and is the first example of the use of 1,3-diketones as acylation agents via C–C bond cleavage.

 Please wait while we load your content...
Please wait while we load your content...